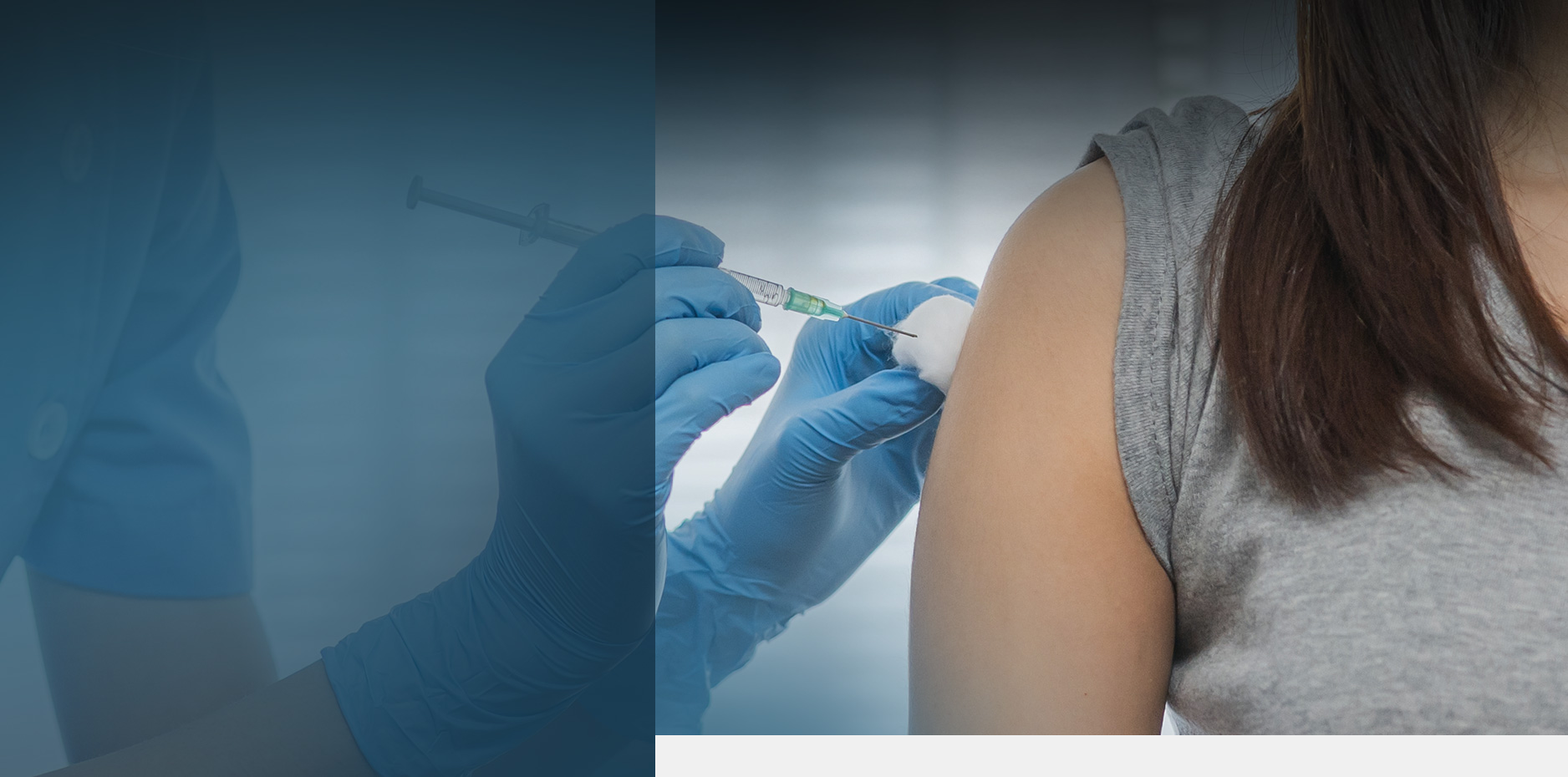
Depo Provera Lawsuit: Birth Control Shot and Brain Tumors
Scientific research uncovered a disturbing connection between Pfizer's injectable birth control medication Depo Provera (medroxyprogesterone acetate) and an increased risk of developing brain tumors called meningiomas. The finding prompted women across the United States to file Depo Provera lawsuits seeking compensation for injuries they believe resulted from using this popular contraceptive. These Depo shot lawsuits are consolidated before Judge M. Casey Rodgers of the Northern District of Florida.
This litigation centers around allegations that Pfizer and other manufacturers failed to adequately warn patients and healthcare providers about the serious risks associated with Depo Provera, despite evidence suggesting they may have known about these dangers for years.
What's particularly concerning is that while European and UK product labels now include warnings about the potential risk of brain tumors, no such warnings exist on products sold in the United States, potentially putting millions of American women at risk without their knowledge.
Wisner Baum is evaluating cases for women who developed brain and spinal tumors after receiving the birth control injection (Depo-Provera or depo-subQ provera 104). Fill out our free case evaluation form to see if you qualify for a lawsuit.
Information on Depo Shot Lawsuits
- Why Is There a Lawsuit for the Depo Shot?
- Current Status of Depo Provera Lawsuits
- Do I Qualify for a Depo Provera Lawsuit?
- Who is Eligible for Depo-Provera Compensation?
- Understanding Depo-Provera and Its Risks
- What Are Meningiomas and Why Are They Dangerous?
- Taking the Next Steps in Your Depo Lawsuit
Why Is There a Lawsuit for the Depo Shot?
The Depo-Provera lawsuits are based on the fact that Pfizer, the company that manufactures the Depo shot, did not provide warnings to patients that the birth control injection can lead to brain tumors. Lawsuits further allege the company knew about the risks for some time.
The allegations against Pfizer and other defendants assert several legal theories of liability. Understanding these claims helps clarify the legal basis for these lawsuits and what plaintiffs must prove to succeed in cases.
Failure to Warn
The primary claim in Depo Provera lawsuits is "failure to warn," which alleges that the manufacturers did not adequately inform patients and healthcare providers about the known risks associated with the product. This legal theory is based on the principle that drug manufacturers have a duty to warn consumers about all known risks associated with their products, especially serious side effects like brain tumors.
Based on scientific studies dating back decades, plaintiffs argue that Pfizer and other defendants knew or should have known about the link between Depo Provera and meningiomas. According to the complaints, the association between progesterone and meningioma has been known or knowable since at least 1983. Despite this knowledge, the manufacturers allegedly failed to include warnings about this risk on their U.S. product labels, depriving patients of the opportunity to make informed decisions about their contraceptive choices.
Strict Product Liability
Many lawsuits also include claims of strict product liability, which holds manufacturers accountable for injuries caused by defective products regardless of whether they were negligent. Under this theory, plaintiffs argue that Depo Provera is defective and unreasonably dangerous when used as intended due to its propensity to cause brain tumors.
The complaints allege that the design of Depo Provera, particularly its high dose of synthetic progestin (150 mg), makes it more dangerous than necessary for effective contraception.
Negligence
Negligence claims focus on the manufacturers' alleged failure to exercise reasonable care in the design, testing, and marketing of Depo Provera. Plaintiffs contend that the defendants breached their duty of care by not conducting adequate long-term studies on the safety of their product, despite early indications that it might increase the risk of meningiomas.
The lawsuits also allege negligence in the manufacturers' post-market surveillance practices, claiming they failed to properly monitor and respond to adverse event reports that might have indicated a connection between Depo Provera and brain tumors.
Fraudulent Concealment
Some lawsuits include claims of fraudulent concealment, alleging that the manufacturers deliberately withheld information about the risks of Depo Provera to protect their profits. These claims assert that the defendants had a duty to disclose the potential dangers of their product but instead chose to conceal this information from patients and healthcare providers.
The allegations of fraudulent concealment are particularly serious because they suggest intentional wrongdoing rather than mere negligence.
Current Status of Depo Provera Lawsuits
The Depo Provera lawsuit is an emerging tort with more and more women becoming aware of the potential link between their brain tumors and their use of this birth control method.
Numerous lawsuits have been filed against Pfizer and other manufacturers, with many more expected in the coming months. These legal actions allege that defendant companies failed to adequately warn patients and healthcare providers about the risk of developing meningiomas, despite evidence suggesting they knew or should have known about these dangers.
On February 7, 2025, the U.S. Judicial Panel on Multidistrict Litigation (JPML) consolidated more than 70 Depo Provera lawsuits into multidistrict litigation (MDL) in the Northern District of Florida under Judge M. Casey Rodgers. Legal experts project that the number of Depo Provera claims could grow substantially, with many thousands of women potentially eligible to file cases nationwide.
Do I Qualify for a Depo Provera Lawsuit?
If you or a family member used Depo-Provera or an authorized generic version and subsequently developed a meningioma, you may be eligible to join the growing litigation against the manufacturers. Understanding whether you qualify for a Depo Provera lawsuit requires considering several important factors that can strengthen your potential claim.
Generally, individuals who may qualify for a Depo Provera lawsuit include those who received multiple Depo Provera injections over time and were later diagnosed with a meningioma.
Research indicates that prolonged use of the birth control shot is associated with a higher risk of developing these brain tumors, so claimants who used the medication for an extended period may have stronger cases. However, each situation is unique, and evaluating the specific circumstances of your case requires professional legal assessment.
To have a viable claim, you should typically meet the following criteria:
- You received at least two injections of Depo-Provera or its generic version (medroxyprogesterone acetate) after 1992, when it received FDA approval for contraceptive use.
- You were subsequently diagnosed with a meningioma (either malignant or benign).
It's important to note that you may still have a valid claim even if you are uncertain whether your meningioma is directly related to your use of Depo Provera. The best way to determine whether you qualify is to contact an attorney and fill out a free case evaluation form. It will take you a few minutes to complete the evaluation, and a member of our legal team will follow up with you to discuss your options.
Who is Eligible for Depo-Provera Compensation?
It is natural for people to want to know whether they are eligible for compensation and what their case might be worth. However, the litigation involving Depo Provera is still in its early stages, making it premature to predict specific settlement amounts.
These lawsuits generally seek compensation for various types of damages experienced by affected individuals. Understanding the potential forms of compensation can help prospective claimants better understand what may be available in successful cases.
Compensatory damages typically form the foundation of recovery in cases like these. These damages aim to compensate plaintiffs for actual losses suffered and may include coverage for medical expenses, both past and future. Given the serious nature of meningiomas, affected individuals often incur substantial costs for diagnostic procedures, surgical interventions, radiation therapy, medication, and rehabilitation services. These medical expenses can quickly become overwhelming, making their recovery through litigation significant for many families.
Beyond medical costs, compensatory damages may also include lost wages and loss of earning capacity when brain tumors and their treatment prevent individuals from working or limit their ability to earn income at previous levels. The physical and emotional suffering associated with meningiomas and their treatment represents another significant category of damages, often referred to as "pain and suffering." This compensation acknowledges the physical pain, emotional distress, and overall decrease in quality of life that often accompanies these conditions.
In cases where the court finds the manufacturer's conduct particularly egregious, punitive damages may also be available. Unlike compensatory damages, which aim to make the plaintiff "whole" again, punitive damages are designed to punish wrongdoers and deter similar behavior in the future.
Understanding Depo-Provera and Its Risks
Depo Provera, commonly known as the "Depo shot," is a progestin-only injectable contraceptive that millions of women have used since receiving FDA approval in 1992. This birth control method involves receiving injections every three months, making it a convenient option for many women seeking long-term contraception.
The contraceptive works by suppressing ovulation, thickening cervical mucus to block sperm, and thinning the uterine lining to prevent implantation. In addition to its use as a contraceptive, the FDA approved DepoSubQ Provera 104 in 2004 as a treatment for endometriosis, expanding its applications beyond birth control. While these mechanisms effectively prevent pregnancy, emerging research suggests they may come with serious health consequences that many users were never warned about.
Is Depo Provera Dangerous?
The most alarming evidence comes from a 2024 study published in the British Medical Journal (BMJ), which analyzed over 18,000 women who underwent surgery for intracranial meningioma between 2009 and 2018. This comprehensive research revealed that prolonged use of Depo Provera resulted in a 555% increased risk of developing these brain tumors compared to non-users.
More specifically, the study found that users of the Depo shot were 5.55 times more likely to be diagnosed with meningiomas. This finding represents a significant health concern, especially considering that numerous studies published in previous decades reached similar conclusions, suggesting manufacturers may have been aware of this risk long before informing the public.
What Are Meningiomas and Why Are They Dangerous?
Intracranial meningiomas are tumors that develop in the protective membranes (meninges) covering the brain and spinal cord. While these tumors are typically classified as "benign" or non-cancerous, their location inside the skull makes them potentially dangerous regardless of their cellular classification. As these tumors grow within the confined space of the skull, they can exert pressure on sensitive brain tissue, leading to serious and sometimes life-threatening complications.
Patients diagnosed with meningiomas often experience a range of debilitating symptoms that can significantly impact their quality of life. These symptoms may include:
- Severe headaches
- Vision problems
- Seizures
- Muscle weakness
- Speech difficulties
- Personality changes
In more advanced cases, cognitive impairment, memory loss, and even life-threatening complications may occur as the tumor continues to grow and press against vital brain structures. The seriousness of these symptoms underscores why proper warnings about the potential health risks are crucial for patients considering their contraceptive options.
Meningioma Treatment and Recovery
Treatment for meningiomas typically involves invasive procedures such as brain surgery (craniotomy), which requires removing part of the skull to access and remove the tumor. Following surgery, patients may require radiation therapy if the tumor cannot be removed entirely or if it recurs.
Recovery from these procedures is often lengthy and challenging, potentially involving extended rehabilitation periods, ongoing medication, and permanent lifestyle changes. Even after successful treatment, patients may continue to experience seizure disorders, neurological issues, anxiety, and depression, emphasizing the life-altering impact of these tumors.
Taking the Next Steps in Your Depo Lawsuit
If you or someone in your family developed a meningioma after using Depo Provera or the Depo shot, it's important to take prompt action to preserve your legal rights. The first step is to contact an experienced attorney for a free and confidential consultation to evaluate whether you may have a valid claim. During this initial consultation, you'll have the opportunity to discuss your specific situation, including your history of Depo Provera use and subsequent medical diagnosis.
If you decide to move forward with a lawsuit, your attorney will guide you through the process of gathering relevant medical records and other evidence to support your claim. This documentation typically includes records of your Depo Provera prescriptions and administration, diagnostic imaging confirming your meningioma, surgical reports, and ongoing treatment records. These materials help establish both your use of the medication and the nature and extent of your injuries.
Time limitations may apply to your case, so it's important to seek legal advice if you believe you may have a claim. Contact Wisner Baum today at (855) 948-5098 or complete a confidential case evaluation form to learn more about your legal options.
Our Case Results

-
$265 Million Settlement Fatal Train Crash
In 2016, Wisner Baum attorney Timothy A. Loranger and six other attorneys in the Plaintiffs’ Management Committee were able to secure a $265 million settlement for victims of the 2015 Amtrak 188 derailment in Philadelphia, one of the largest in the U.S. for 2016.
-
$3.5 Million Settlement Fatal Train Crash
Wisner Baum secured a $3.5 million settlement on behalf of an individual who died on a train.
-
$2 Million Settlement Fatal Train Crash
Wisner Baum obtained a $2 million settlement on behalf of a passenger who died on a train.
-
$2.8 Million Settlement Wrongful Death
Wisner Baum obtained a $2.8 million wrongful death settlement for a train passenger.

Client-Focused Representation
REVIEWS & TESTIMONIALS
We believe our track record speaks for itself. But you don’t have to take our word for it. See what our clients have to say about working with us.
-
"I Can’t Imagine a Better Law Firm"
Multiple lawyers recommended Wisner Baum to me and I have been consistently impressed with the quality of their work.
- Best Law Firms Survey -
"They Are About Changing the Systems..."
Wisner Baum are not only amazing attorneys but more importantly, they are activists. They are about changing the systems which got us into trouble in the first place. They understand their role in the process of making change.
- Kim Witczak -
"Top Legal Minds in the Country"
The Wisner Baum firm has some of the top legal minds in the country; they are driven, determined, trustworthy, ethical and passionate.
- From Best Lawyers® Best Law Firms





